Doxorubicin
Doxorubicin, trade name Adriamycin, is a potent chemotherapy medication used in the treatment of various cancers including breast cancer, leukaemia and lymphoma (Kumar et al., 2014). Doxorubicin belongs to the anthracycline class of drugs, characterised by their ability to interfere with DNA replication and RNA synthesis (Kumar et al., 2014; Tewey et al.., 1984). Initially isolated from the bacterium Streptomyces peucetius var. caesius in the 1960s, it has become an important drug against cancer (Arcamone et al., 1969). However, its clinical application comes with various side effects, including heart toxicity, nephrotoxicity and bone marrow suppression (Peter et al., 2022). In this article, the structure of doxorubicin, its derivatives, its mode of action and its applications are discussed.
Structure of Doxorubicin
Anthracycline antibiotic structures are based on the anthracyclinone core with quinones, and other substituents on the resonating ring structure (Bachur, 2002).
Doxorubicin has a chemical structure that consists of a tetracyclic aglycone core with an anthraquinone nucleus (Figure 1) , and a four cyclohexane chain conjugated to a daunosamine sugar moiety (Figure 2) via a glycosidic linkage at position 7 (Agrawal, 2007). This structure imparts doxorubicin with its characteristic red colour and significant cytotoxic activity against a wide range of cancers (Agrawal, 2007). Doxorubicin is also an electron acceptor with high charge delocalisation, due to its polarity (Sikora et al., 2022).

Figure 1
Wire frame model of Anthraquinone.
Created using MarvinJS.
The aglycone is made up of a tetracyclic ring with adjacent quinone-hydroquinone groups in rings B and C, a methoxy substituent on carbon 4 in ring D and a short side chain containing a carbonyl that ends with a primary alcohol in carbon 9 (Figure 2). The sugar contains a 3-amino-2,3,6-trideoxy-L-fucosyl moiety and is bound by a glycosidic bond (Minotti, 2004).

Figure 2
Labelled structure of doxorubicin.
Created using MarvinJS.
The anthracycline ring system of doxorubicin is highly planar due to the presence of four aromatic rings fused together, contributing to its DNA intercalation ability and inhibition of topoisomerase II (Cutts et al., 2005; Gewirtz et al., 1999). This is achieved through interactions with covalent and non-covalent bonds of the planar aromatic and the heteroaromatic rings of the drug (Khan & Akhtar, 2022; Graves & Velea, 2000).
Furthermore, doxorubicin’s structure contains a quinone moiety which is essential for its mechanism of action. Quinone redox cycling generates reactive oxygen species (ROS), leading to oxidative stress and DNA damage within cancer cells (Thorn et al., 2011).
Mode of Action
The effectiveness of doxorubicin in treating cancer stems from its ability to insert itself between the strands of deoxyribonucleic acid (DNA), hinder the activity of topoisomerase II, disturb mitochondrial function and enhance the production of free radicals, leading to oxidative damage (Kciuk et al., 2023).
DNA Intercalation
The main mode of action of doxorubicin revolves around its capacity to intercalate within the base pairs of DNA, leading to the fragmentation of DNA strands and the suppression of DNA synthesis. Doxorubicin achieves DNA intercalation by forming hydrogen bonds with guanines located in neighbouring GC base pairs (Figure 3) (Kciuk et al., 2023). The six-membered daunosamine sugar moiety occupies the minor groove of the DNA and interacts with the surrounding base pairs immediately flanking the intercalation site. Meanwhile, the planar aromatic chromophore segment of doxorubicin inserts itself between two adjacent base pairs of DNA, effectively intercalating within the DNA helix (Binaschi et al., 2001; Js & Rao, 2012). The intercalation of doxorubicin into DNA hinders the activity of the enzyme topoisomerase II. Topoisomerase II normally regulates DNA coiling by inducing temporary double-stranded breaks in DNA and then repairing them by re-ligation (Lee et al., 2023; Shan, 2013). After breaking the DNA chain, doxorubicin stabilises the topoisomerase II complex and prevents the DNA double helix from resealing. Consequently, this leads to positive supercoiling of the DNA helix, halting the replication process and causing DNA damage. This damage triggers activation of the p53 pathway, resulting in G1 and G2 cell-cycle arrest, and ultimately, apoptosis (Kciuk et al., 2023; Perego et al., 2001; Shan, 2013). Topoisomerase II plays a critical role in the survival and proliferation of cancer cells; therefore, its inhibition and subsequent induction of DNA damage are essential, given the heightened susceptibility of cancer cells to DNA strand breaks compared to healthy cells (Moiseeva, 2019; Van Der Zanden et al., 2020).

Figure 3
An illustration showing doxorubicin (DOX) intercalating with GC base pairs in the minor groove.
Created using Biorender.com
Production of free radical
Another mechanism of action of doxorubicin involves the production of free radicals and their subsequent harm to cellular membranes, proteins and DNA (Gewirtz, 1999; Thorn et al., 2011). doxorubicin directly binds to cardiolipin on the inner mitochondrial membrane, triggering a cascade of reactions which generate reactive oxygen species (ROS). This process occurs through multiple pathways, including peroxisomal metabolism, the action of catabolic oxidases and electron transport chain (ETC) activity (Kciuk et al., 2023; Sarniak et al., 2016). DOX undergoes oxidation to form semiquinone, an unstable metabolite. This semiquinone is then converted back to doxorubicin, releasing reactive oxygen species (ROS) such as superoxide anions (O2−) and hydrogen peroxide (H2O2) in the process (Figure 4). These ROS can further generate free radicals, contributing to oxidative stress within the cell (Doroshow, 1986; Moiseeva, 2019; Van Der Zanden et al., 2020). While ROS typically act as cellular messengers in redox signalling events at low concentrations, an overproduction of ROS can induce DNA damage by radicals interacting with DNA bases and the sugar-phosphate backbone. Unrepaired damage can lead to apoptosis, cell-cycle arrest, and senescence. Moreover, ROS can also lead to lipid peroxidation and membrane damage, DNA damage and oxidative stress (Doroshow, 1986; Kciuk et al., 2023).

Figure 4
Illustration of the formation of reactive oxygen species by doxorubicin
Created using Biorender.com
Side effects
While doxorubicin demonstrates considerable efficacy as an anticancer agent, its nonspecific action, limited solubility and poor distribution serve as major drawbacks and pose significant challenges in its clinical use, ultimately resulting in substantial side effects (Mohan et al., 2021; Van Der Zanden et al., 2020).
The primary side effect of doxorubicin is cardiotoxicity, which develops cumulatively, causing the administration of doxorubicin to be limited to a maximum of six to eight treatment cycles (Lefrak et al., 1973). Doxorubicin-induced cardiac toxicity stems from heightened cardiac myocyte apoptosis, reduced expression of cardiac-specific genes and oxidative stress. Acute cardiac toxicity presents as arrhythmias, left ventricular dysfunction or reversible myopericarditis, typically occurring within days of doxorubicin administration. This adverse event affects approximately 11% of patients receiving the medication. Doxorubicin-induced irreversible cardiomyopathy can manifest within a few months following the conclusion of treatment. However, cases of its occurrence have also been documented up to twenty years after the termination of treatment (Johnson-Arbor & Dubey, 2023). In addition to dose-related adverse effects, the inadequate delivery of doxorubicin to the targeted tumour can also lead to toxicity in the kidneys, liver and brain (Peter et al., 2022).
Derivatives
The alteration of a compound's chemical makeup leads to a change in its pharmacokinetic properties. These derivatives can be useful for developing better anticancer medications by increasing retention time, reducing toxicity, and minimising adverse drug effects. Derivatives of doxorubicin have been developed and have been reported to be promising anticancer agents. In addition, techniques such as encapsulation of the drug in nanoparticles or in liposomes, have been investigated to reduce the adverse effects of doxorubicin.
Doxorubicin possesses two functional groups (Figure 5), comprising a hydroxyl group and an amide group, that can be used for the synthesis of derivatives.

Figure 5
Wireframe diagram of doxorubicin highlighting the functional groups commonly modified during the production of doxorubicin derivatives.
1 indicates the hydroxyl group, 2 indicates the amide group.
Created using MarvinJS
In 2017, Choi et al. conducted a study where they synthesised four derivatives, two with alterations at the amino group (Lipo-Dox C and Lipo-Dox D) and two with alterations at the hydroxyl group (Lipo-Dox A and Lipo-Dox B), to form four different cholesteryl-doxorubicin derivatives (Figure 6). The cytotoxicity of these compounds was tested using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay which showed that only the derivatives with modified hydroxyl groups have anticancer activity. Studies have consistently shown that modifications to the amide group reduce doxorubicin efficacy, implying that the free ‘-NH2’ is essential for doxorubicin’s anticancer activity
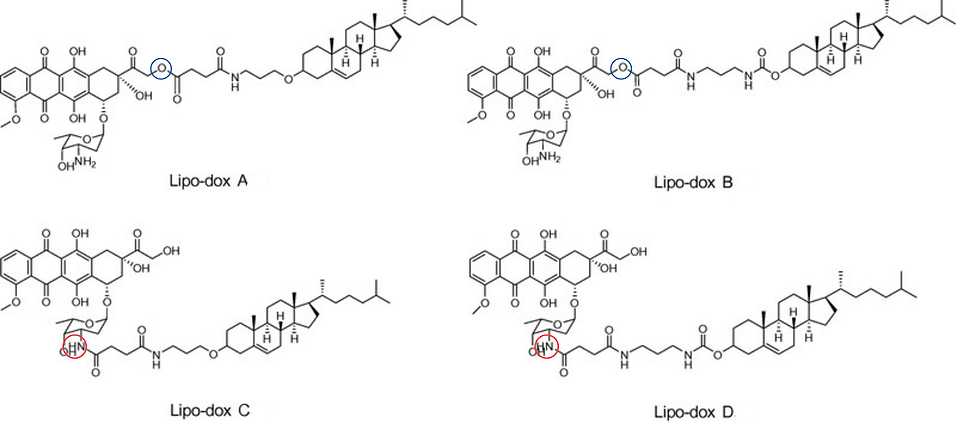
Figure 6
Lipo-dox derivatives synthesised by the Choi et al. 2017 study.
Lipo-dox A and Lipo-dox B were altered at the hydroxyl group, highlighted by a blue circle. Lipo-dox-C and Lipo-dox-D were altered at the amide group, highlighted by a red circle.
Adapted from Choi, J. S., Doh, K. O., Kim, B. K., & Seu, Y. B. (2017). Synthesis of cholesteryl doxorubicin and its anti-cancer activity. Bioorganic & Medicinal Chemistry Letters, 27(4), 723–728. https://doi.org/10.1016/j.bmcl.2017.01.048
The effective modified compounds (lipo-dox A and lipo-dox B) remained in the cell for a longer period than doxorubicin, potentially because of their lipophilic characteristics (Choi et al., 2017)
Fatty acid chains have been added to doxorubicin with the aim of extending its half life and improving its bioavailability. The first generation of doxorubicin-fatty acyl derivatives were synthesised through the modification of the amino group. While these compounds had enhanced lipophilicity when compared to doxorubicin, they had a reduction in anticancer activity due to the disruption of the amide group (Damodar et al., 2014). The second generation of fatty acyl derivatives were synthesised through modification of the hydroxyl group. These doxorubicin-fatty acyl derivatives were tested to identify the one with the highest antiproliferative activity, which was subsequently subjected to further experimentation. It had a higher cellular uptake and was more cytotoxic than doxorubicin. The increased cytotoxicity was attributed to the derivatives ability to remain in the cell. It also exhibited much higher topoisomerase inhibitory activity than doxorubicin (Chhikara et al., 2012).
Doxorubicin has also been conjugated with other fatty acids, resulting in varying degrees of effectiveness in reducing its adverse effects. DOX-hyd-LNA (Figure 7) , developed by Liang et al., emerged as a notable derivative with higher cytotoxicity to certain tumour cells than doxorubicin. In addition, compared to other derivatives, DOX-hyd-LNA released a greater amount of doxorubicin, following cellular uptake (Liang et al., 2014).

Figure 7
Wireframe diagram of DOX-hyd-LNA, a doxorubicin and fatty acid derivative synthesised by Liang et al., 2014.
Reproduced with permission from Adapted from Liang, C. H., Ye, W. L., Zhu, C. L., Na, R., Cheng, Y., Cui, H., Liu, D. Z., Yang, Z. F., & Zhou, S. Y. (2014). Synthesis of doxorubicin α-linolenic acid conjugate and evaluation of its antitumor activity. Molecular Pharmaceutics, 2014, 1378–1390. https://doi.org/10.1021/mp4004139. Copyright 2014 American Chemical Society
As mentioned, a prominent side effect of doxorubicin is cardiotoxicity, caused by oxidative stress. To reduce this adverse effect, compounds with antioxidant activity (ferulic acid, caffeic acid and quercetin) were combined with doxorubicin to create hybrid compounds (Chegaev et al., 2013). While these compounds decreased oxidative stress, it was the doxorubicin-quercetin derivative that demonstrated the most potent cytotoxic effect, attributed to its low water solubility and gradual release of doxorubicin (Alrushaid et al., 2017).
Doxorubicin hybrids have also been synthesised using combretastatin A4, a photoresponsive prodrug, a medication that can be activated or controlled by exposure to light. Photo-removable groups serve as drug transporters, safeguarding the drug until it reaches its intended destination. Upon exposure to a specific wavelength of light, they release the drug, facilitating targeted delivery. The resulting hybrid exhibits increased cytotoxicity in comparison to doxorubicin and also displays the highest efficacy when used in combination therapy (Liu et al., 2019).
Doxorubicin derivatives bearing tumour selective groups have also been synthesised, for example, the anti-estrogen doxorubicin bioconjugate (Figure 8). This derivative shows increased specificity for certain breast cancers and reduces doxorubicin’s side effects while enhancing its efficacy. This compound exhibited a much higher cytotoxicity than doxorubicin in estrogen receptor (ER) positive breast cancer cells and equipotent cytotoxicity to doxorubicin in ER negative breast cancer cells (Dao et al., 2012).

Figure 8
Wireframe model of anti-estrogen doxorubicin bioconjugate, synthesised by Dao et al., 2012.
Adapted from Dao, K. L., Sawant, R. R., Hendricks, J. A., Ronga, V., Torchilin, V. P., & Hanson, R. N. (2012). Design, synthesis, and initial biological evaluation of a steroidal anti-estrogen–doxorubicin bioconjugate for targeting estrogen receptor expressing cancers. Bioorganic & Medicinal Chemistry Letters, 22(3), 1425–1428. https://doi.org/10.1016/j.bmcl.2011.12.078
In-silico computational modelling is increasingly being used to predict drug behaviour, carry out molecular dynamic simulations and understand molecule interactions with greater efficacy. Many research groups are now using these techniques to explore ways to load doxorubicin into nanoparticles, however, there's been little research on using these technologies to synthesise new derivatives and hybrids of doxorubicin.
Applications
Doxorubicin is a common drug in the treatment of breast cancer, being a common part of combination chemotherapy regimens. Its application has been associated with improved survival rates particularly in patients with aggressive or metastatic breast cancer. Research indicates that the use of doxorubicin in therapy significantly enhances the efficacy of treatment therefore leading to better outcomes for patients facing this challenging disease (Chabner & Roberts, 2005). For example, the addition of doxorubicin to standard chemotherapy has been associated with a 20% reduction in the risk of breast cancer recurrence and a 15% decrease in the risk of death therefore highlighting its impact on treatment outcomes (Early Breast Cancer Trialists’ Collaborative Group, 2012).
Doxorubicin’s ability to interfere with the DNA replication of cancer cells makes it a powerful drug in the treatment of Hodgkin’s and non-Hodgkin’s lymphoma (Chabner & Roberts, 2005). Its role in combination therapies has been essential for ensuring high cure rates for these types of cancers. A landmark study demonstrated that the ABVD (adriamycin (DOX), bleomycin, vinblastine, dacarbazine) regimen resulted in a five-year survival rate of approximately 85%, significantly higher than alternative treatments (Connors et al., 2017). This regimen’s success demonstrates doxorubicin’s contribution in the effective management of Hodgkin’s lymphoma.
Studies have shown the importance of doxorubicin in the management and treatment of sarcomas including both soft tissue and bone sarcomas, noting its contribution to improved survival and better disease control. In this context, doxorubicin not only helps to reduce tumour size but also enhances the efficacy of subsequent treatments such as surgery or radiation (Tacar, Sriamornsak, & Dass, 2013). Its use in paediatric patients with osteosarcoma was shown in the EURAMOS-1 trial which explored the outcomes of adding doxorubicin to standard treatment protocols for osteosarcoma. The study found that doxorubicin, when used in conjunction with methotrexate, cisplatin and ifosfamide, contributed to a significant improvement in event-free survival among children and young adults (Marina et al., 2016). This indicates doxorubicin’s versatility and efficacy in treating both adult and paediatric sarcoma patients, highlighting its broad utility across different patient populations.
Conclusion
In conclusion, doxorubicin remains an important component in the fight against cancer, demonstrating significant efficacy across a range of malignancies. Its ability to disrupt DNA replication and induce apoptosis has proven effective in shrinking tumours and extending survival rates. However, the clinical use of doxorubicin is often limited by its adverse effects. Thus, the challenge lies in striking a balance between its therapeutic benefits and potential harm. Ongoing research attempts are focused on finding combination therapies, and targeted delivery mechanisms to minimise its toxicity.
References
Agrawal, K. (2007). Doxorubicin. xPharm: The Comprehensive Pharmacology Reference, 1–5. https://doi.org/10.1016/b978-008055232-3.61650-2
Alrushaid S., Zhao Y., Sayre C.L., Maayah Z.H., Laird Forrest M., Senadheera S.N., Chaboyer K., Anderson H.D., El-Kadi A.O.S., Davies N.M. (2017). Mechanistically elucidating the in vitro safety and efficacy of a novel doxorubicin derivative. Drug Delivery and Translational Research, 7, 582–597. https://doi.org/10.1007/s13346-017-0379-2.
Arcamone, F., Franceschi, G., Penco, S., & Selva, A. (1969). Adriamycin (14-hydroxydaunomycin), a novel antitumor antibiotic. Tetrahedron Letters, (13), 1007–1010. https://doi.org/10.1016/s0040-4039(01)97723-8
Bachur, N. R. (2002). Antracyclines. Encyclopedia of Cancer (Second Edition), 57-61. https://doi.org/10.1016/B0-12-227555-1/00006-X
Binaschi, M., Bigioni, M., Cipollone, A., Rossi, C., Goso, C., Maggi, C. A., Capranico, G., & Animati, F. (2001). Anthracyclines: selected new developments. Current Medicinal Chemistry, 1(2), 113–130. https://doi.org/10.2174/1568011013354723
Chabner, B. A., & Roberts, T. G. (2005). Timeline: Chemotherapy and the war on cancer. Nature Reviews Cancer, 5(1), 65–72. https://doi.org/10.1038/nrc1529
Chegaev, K., Riganti, C., Rolando, B., Lazzarato, L., Gazzano, E., Guglielmo, S., Ghigo, D., Fruttero, R., & Gasco, A. (2013). Doxorubicin-antioxidant co-drugs. Bioorganic & Medicinal Chemistry Letters, 23(20), 5307–5310. https://doi.org/10.1016/j.bmcl.2013.07.070
Chhikara, B. S., Mandal, D., & Parang, K. (2012). Synthesis, anticancer activities, and cellular uptake studies of lipophilic derivatives of doxorubicin succinate. Journal of Medicinal Chemistry, 55(3), 1500–1510. https://doi.org/10.1021/jm201653u
Choi, J. S., Doh, K. O., Kim, B. K., & Seu, Y. B. (2017). Synthesis of cholesteryl doxorubicin and its anti-cancer activity. Bioorganic & Medicinal Chemistry Letters, 27(4), 723–728. https://doi.org/10.1016/j.bmcl.2017.01.048
Connors, J. M., Jurczak, W., Straus, D. J., Ansell, S. M., Kim, W. S., Gallamini, A., Younes, A., Alekseev, S., Illés, Á., Picardi, M., Lech-Maranda, E., Oki, Y., Feldman, T., Smolewski, P., Savage, K. J., Bartlett, N. L., Walewski, J., Chen, R., Ramchandren, R., & Zinzani, P. L. (2018). Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin’s Lymphoma. New England Journal of Medicine, 378(4), 331–344. https://doi.org/10.1056/nejmoa1708984
Cutts, S., Nudelman, A., Rephaeli, A., & Phillips, D. (2005). The Power and Potential of Doxorubicin-DNA Adducts. IUBMB Life (International Union of Biochemistry and Molecular Biology: Life), 57(2), 73–81. https://doi.org/10.1080/15216540500079093
Damodar, G., Smitha, T., Gopinath, S., Vijayakumar, S., & Rao, Y. A. (2014). An evaluation of hepatotoxicity in breast cancer patients receiving injection Doxorubicin. Annals of Medical and Health Sciences Research, 4(1), 74–79. https://doi.org/10.4103/2141-9248.126619
Dao, K. L., Sawant, R. R., Hendricks, J. A., Ronga, V., Torchilin, V. P., & Hanson, R. N. (2012). Design, synthesis, and initial biological evaluation of a steroidal anti-estrogen–doxorubicin bioconjugate for targeting estrogen receptorexpressing cancers. Bioorganic & Medicinal Chemistry Letters, 22(3), 1425–1428. https://doi.org/10.1016/j.bmcl.2011.12.078
Dimanche-Boitrel, M. T., Meurette, O., Rebillard, A., & Lacour, S. (2011). Role of Early Plasma Membrane Events in Chemotherapy-Induced Cell Death. Drug Resistance Updates, 14(1), 22–31. https://doi.org/10.1016/j.drup.2010.11.003
Doroshow, J. H. (1986).Role of hydrogen peroxide and hydroxyl radical formation in the killing of Ehrlich tumor cells by anticancer quinones. Proceedings of the National Academy of Sciences of the United States of America, 83(12), 4514–4518. https://doi.org/10.1073/pnas.83.12.4514
Early Breast Cancer Trialists’ Collaborative Group. (2012). Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100 000 women in 123 randomised trials. The Lancet, 379(9814), 432–444. https://doi.org/10.1016/s0140-6736(11)61625-5
Gewirtz, D. A. (1999). A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochemical Pharmacology, 57(7), 727–741. https://doi.org/10.1016/s0006-2952(98)00326-3
Graves, D. E., & Velea, M. L. (2000). Intercalative Binding of Small Molecules to Nucleic Acids. Current Organic Chemistry, 4(9), 915–929. https://doi.org/10.2174/1385272003375978
Hortobagyi, G. N. (1997). Anthracyclines in the treatment of cancer. An overview. Drugs, 54(Suppl 4), 1–7. https://doi.org/10.2165/00003495-199700544-00002
Johnson-Arbor, K., & Dubey, R. (2023). Doxorubicin. StatPearls - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK459232/
Js, D., & Rao, V. (2012). Current and Proposed biomarkers of anthracycline Cardiotoxicity in Cancer: Emerging opportunities in oxidative damage and autophagy. Current Molecular Medicine, 12(6), 763–771. https://doi.org/10.2174/156652412800792561
Kciuk, M., Gielecińska, A., Mujwar, S., Kołat, D., Kałuzińska, Ż., Çeli̇K, İ., & Kontek, R. (2023). Doxorubicin—An Agent with Multiple Mechanisms of Anticancer Activity. Cells, 12(4), 659. https://doi.org/10.3390/cells12040659
Khan, S. A., & Akhtar, M. J. (2022). Structural modification and strategies for the enhanced doxorubicin drug delivery. Bioorganic Chemistry, 120. https://doi.org/10.1016/j.bioorg.2022.105599
Kumar, A., Gautam, B., Dubey, C., Tripathi, P. K. (2014). A review: role of doxorubicin in treatment of cancer. International Journal of Pharmaceutical Sciences and Research, 5(10). 10.13040/IJPSR.0975-8232.5(10).4117-28
Lee, J., Choi, M., & Song, I. (2023). Recent advances in doxorubicin formulation to enhance pharmacokinetics and tumor targeting. Pharmaceuticals, 16(6), 802. https://doi.org/10.3390/ph16060802
Lefrak, E. A., Piťha, J., Rosenheim, S., & Gottlieb, J. A. (1973). A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer, 32(2), 302–314. https://doi.org/10.1002/1097-0142(197308)32:2
Lemke, T. L., & Williams, D. A. (2012). Foye's Principles of Medicinal Chemistry (7th ed.). Wolters Kluwer Health/Lippincott Williams & Wilkins.
Liang, C. H., Ye, W. L., Zhu, C. L., Na, R., Cheng, Y., Cui, H., Liu, D. Z., Yang, Z. F., & Zhou, S. Y. (2014). Synthesis of doxorubicin α-linolenic acid conjugate and evaluation of its antitumor activity. Molecular Pharmaceutics, 2014, 1378–1390. https://doi.org/10.1021/mp4004139
Liu W., Liang L., Zhao L., Tan H., Wu J., Qin Q., Gou X., Sun X. (2019) Synthesis and characterization of a photoresponsive doxorubicin/combretastatin A4 hybrid prodrug. Bioorganic & Medicinal Chemistry Letters, 29, 487–490. https://doi.org/10.1016/j.bmcl.2018.12.017.
Lorusso, D., Di Stefano, A., Carone, V., Fagotti, A., Pisconti, S., & Scambia, G. (2017). Pegylated liposomal doxorubicin-related palmar-plantar erythrodysesthesia ('hand-foot' syndrome). Annals of Oncology, 18(7), 1159–1164. https://doi.org/10.1093/annonc/mdm093
Marina, N. M., Smeland, S., Bielack, S. S., Bernstein, M., Jovic, G., Krailo, M. D., Hook, J. M., Arndt, C., van den Berg, H., Brennan, B., Brichard, B., Brown, K. L. B., Butterfass-Bahloul, T., Calaminus, G., Daldrup-Link, H. E., Eriksson, M., Gebhardt, M. C., Gelderblom, H., Gerss, J., & Goldsby, R. (2016). Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. The Lancet. Oncology, 17(10), 1396–1408. https://doi.org/10.1016/S1470-2045(16)30214-5
Mattioli, R., Ilari, A., Colotti, B., Mosca, L., Fazi, F. & Colotti, G. (2023). Doxorubicin and other anthracyclines in cancers: Activity, chemoresistance and its overcoming. Molecular aspects of Medicine, 93. https://doi.org/10.1016/j.mam.2023.101205
Minotti, G., Menna, P., Salvatorelli, E., Cairo, G., & Gianni, L. (2004). Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacological Reviews, 56(2), 185–229. https://doi.org/10.1124/pr.56.2.6
Mohan, U. P., Pichiah, P. T., Iqbal, S. T. A., & Arunachalam, S. (2021). Mechanisms of doxorubicin-mediated reproductive toxicity – A review. Reproductive Toxicology, 102, 80–89. https://doi.org/10.1016/j.reprotox.2021.04.003
Moiseeva, A. A. (2019). Anthracycline derivatives and their anticancer activity. Ineos Open, 2(1). https://doi.org/10.32931/io1902r
Perego, P., Corna, E., De Cesare, M., Gatti, L., Polizzi, D., Pratesi, G., Supino, R., & Zunino, F. (2001). Role of apoptosis and Apoptosis-Related genes in cellular response and antitumor efficacy of anthracyclines. Current Medicinal Chemistry, 8(1), 31–37. https://doi.org/10.2174/0929867013373994
Peter, S., Alven, S., Maseko, R. B., & Aderibigbe, B. A. (2022). Doxorubicin-Based Hybrid Compounds as Potential Anticancer Agents: A Review. Molecules (Basel, Switzerland), 27(14), 4478. https://doi.org/10.3390/molecules27144478
Sarniak, A., Lipińska, J., Tytman, K., & Lipińska, S. (2016). Endogenous mechanisms of reactive oxygen species (ROS) generation. PostȩPy Higieny I Medycyny DośWiadczalnej, 70, 1150–1165. https://doi.org/10.5604/17322693.1224259
Shan, L. (2013). 99mTc-Labeled doxorubicin. Molecular Imaging and Contrast Agent Database (MICAD) - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK115432
Sharma, A., Jaiswal, S. K., Chaudhary, P., & Mittal, A. (2014). Role of ferroptosis in cell death and its implications in cancer therapy: a review. Cancer Biology & Therapy, 15(12), 1463–1472. https://doi.org/10.4161/15384047.2014.972233
Sikora T, Morawska K, Lisowski W, Rytel P, Dylong A. (2002). Application of Optical Methods for Determination of Concentration of Doxorubicin in Blood and Plasma. Pharmaceuticals 15(2):112. https://doi.org/10.3390/ph15020112
Sledge, G. W., Neuberg, D., Bernardo, P., Ingle, J. N., Martino, S., Rowinsky, E. K., & Wood, W. C. (2003). Phase III trial of doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as front-line chemotherapy for metastatic breast cancer: An Intergroup trial (E1193). Journal of Clinical Oncology, 21(4), 588–592. https://doi.org/10.1200/jco.2003.08.013
Tacar, O., Sriamornsak, P., & Dass, C. R. (2012). Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. Journal of Pharmacy and Pharmacology, 65(2), 157–170. https://doi.org/10.1111/j.2042-7158.2012.01567.x
Tewey, K. M., Rowe, T. C., Yang, L., Halligan, B. D., & Liu, L. F. (1984). Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science, 226(4673), 466–468. https://doi.org/10.1126/science.6093249
Thorn, C. F., Oshiro, C., Marsh, S., Hernandez-Boussard, T., McLeod, H., & Klein, T. E. (2011). Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenetics and genomics, 21(7), 440-446. Van Der Zanden, S. Y., Qiao, X., & Neefjes, J. (2020). New insights into the activities and toxicities of the old anticancer drug doxorubicin. The FEBS Journal, 288(21), 6095–6111. https://doi.org/10.1111/febs.15583
Weiss, R. B. (1992). The anthracyclines: will we ever find a better doxorubicin? Seminars in Oncology, 19(6), 670–686.
Zunino, F., Capranico, G., & Di Marco, A. (1988). The role of the sugar moiety in the pharmacological activity of anthracyclines: development of new derivatives. Anti-Cancer Drug Design, 3(1), 25–39.
