
L-Arabinose
Introduction
L-Arabinose is a naturally occurring carbohydrate that was first isolated as a component of gum arabic, after which it was named (Fehér, 2018). This biomolecule is found primarily within the cell walls of plants as one of the building blocks of its many biopolymers such as pectin or hemicellulose, and its metabolism is especially important to certain bacterial strains such as Escherichia coli. In recent years, L-arabinose has shown promise within the chemical, pharmaceutical and biotechnological industries (Crozier et al., 2021; Fehér, 2018).
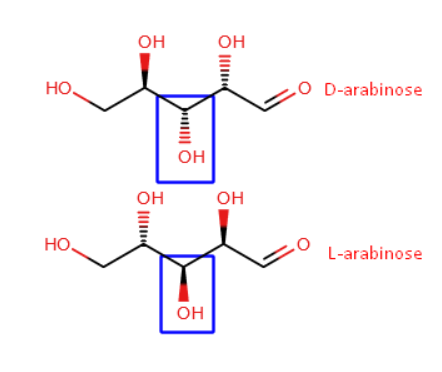
Arabinose is a monosaccharide, specifically in the aldopentose family. This means that it is composed of a polyhydroxy aldehyde with a five-carbon backbone (Asatkar & Basak, 2023). This molecular structure and arrangement has allowed for a level of stereoisomerism to take place within arabinose leading to the formation of L-arabinose and D-arabinose (Asatkar & Basak, 2023). Interestingly, while most monosaccharides are primarily found in their D- form, arabinose is an exception, where it is found in its L- form, particularly as a cyclic furanose (Asatkar & Basak, 2023; Seiboth & Metz, 2011).
Figure 1. Image showing the structures of 2 stereoismers of arabinose: L-arabinose and D-arabinose
With this in mind, the remainder of this article will delve into the metabolism of L-arabinose, the effects it has on human health, particularly in relation to glucose and insulin metabolism, as well as the role it plays for the gut microbiome.
Metabolism of L-Arabinose
Metabolism of L-Arabinose in Humans
Recent studies on the metabolism of L-arabinose have shown promising results, indicating a reduction in peak and post-prandial blood plasma glucose concentrations. This discovery holds significant potential in combating diabetes and related disorders (Krog-Mikkelsen et al., 2011). Nonetheless, researching this topic in humans has been extremely challenging as L-arabinose is poorly absorbed in the body. Furthermore, the metabolism of the sugar has only been characterised in fungi and yeast and the mechanisms behind its mode of action and specific metabolic pathways in humans currently remains unknown (Adamczyk et al., 2023; Seiboth & Metz, 2011).
Despite this knowledge gap, a case study by Shaw et al., (1995).has presented an interesting case of L-arabinosuria. The condition refers to the presence of L-arabinose in urine. This was found via laboratory testing in which a drastic amount of L-arabinose and its metabolite L-arabitol was present in urine; both these sugars were also elevated in plasma and cerebrospinal fluid. L-arabitol is formed when L-arabinose reductase metabolises L-arabinose. However it is due to a mutation in downstream L-arabitol dehydrogenase which induces an accumulation of L-arabitol although the specific changes in the enzyme have not been characterised. Figure 1 illustrates a proposed biochemical pathway in humans which is based on previous findings and describes that on ingestion of L-arabinose it is converted to L-arabitol but is unable to be eliminated via the pentose phosphate pathway due to the aforementioned mutation in L-arabitol dehydrogenase. This would in turn result in the accumulation of L-arabitol (Shaw et al., 1995).
Figure 2. Diagram of proposed pathway of L-arabinose metabolism in humans. In the wild type L-arabitol dehydrogenase the enzyme should convert L-arabitol to L-ribulose and L-xyulose following the previous conversion of L-arabinose to L-arabitol. The metabolites of L-arabitol dehydrogenase are then eliminated via the pentose phosphate pathway. In L-arabinosuria however, a defect in the enzyme prevents its function and as such subsequent conversion of the molecule is prevented thus leading to an accumulation of L-arabitol to potentially toxic concentrations.
The lack of studies regarding the metabolism of L-arabinose has led to the only proposed map being based on model organisms and may not be accurate for humans. Furthermore, diagnosis of the disease is extremely difficult due to its similarity with alimentary pentosuria, a condition where someone ingests excess amounts of L-xyulose through eating large amounts of fruits. Furthermore the rarity of the disease also makes it much more difficult to study with only one other existing case in literature where the stereochemistry of the molecule was not resolved (Onkenhout et al., 2002). Additionally the presence of L-arabinose reductase in humans is dubious. As of the time of writing, the gene which codes for the enzyme has been identified in eukaryotic organisms and not in humans (Mojzita et al., 2010). As such it may be possible that L-arabinosuria is caused via another unknown mechanism. This is further exemplified by the fact that arabinose is not readily absorbed in humans (Mojzita et al., 2010).
Effect of L-Arabinose on Glucose and Insulin Homeostasis
L-Arabinose’s Role in Maintaining Glucose and Insulin Homeostasis
More recently, research efforts have focused on uncovering the potential health benefits of L-arabinose. Recent findings suggest its ability to reduce post-prandial blood plasma glucose concentrations, a crucial aspect in managing Diabetes Mellitus (DM).
Diabetes Mellitus (DM) comprises a series of metabolic disorders that are primarily characterized by persistently elevated blood glucose levels. These disorders result from a lack of insulin secretion, its reduced effectiveness as an anabolic hormone, or a combination of both. This disrupts the body’s ability to properly metabolise carbohydrates, fats and proteins over time (Puchulu, 2018). Metabolic disorders in people with DM result from inadequate insulin levels, where either an appropriate response to elevated blood glucose levels is not achieved or resistance exists at the molecular level. This affects insulin receptors, signalling pathways, effector enzymes, and genes in the target tissues, and primarily affects skeletal muscle, adipose tissue, and the liver (Kharroubi & Darwish, 2015).
Worldwide, the incidence of these diseases is around 9%, with the regions of the Middle East and North Africa having the highest rates among adults at 12% (Puchulu, 2017). Incidence rates are rising steadily, and forecasts predict that the global prevalence of DM will reach around 12% by 2035 (Kharroubi & Darwish, 2015). A separate study assessed the global impact of diabetes in adults that are between 20 to 79 years old and estimated the affected population at 415 million, a figure that is expected to rise to 642 million by 2040 (Ogurtsova et al., 2017). Recent data from the International Diabetes Federation (2019) shows 1 in 11 adults worldwide are struggling with diabetes, with half of them undiagnosed. Furthermore, a fifth of those affected are 65 years old and over.
This blog will primarily focus on type 2 diabetes, with a particular emphasis on the implications of L-arabinose in relation to this disease. We will delve into how L-arabinose might affect different aspects of T2DM and its management.
T2DM, formerly known as non-insulin-dependent diabetes or adult-onset diabetes, results from a combination of insulin resistance and impaired insulin secretion by the pancreatic β-cells (Bailes, 2002; Kaul et al., 2012; Kerner & Brückel, 2014). Its incidence increases with age (Kaul et al., 2012). Treatment options often include lifestyle changes, such as increased physical activity and weight loss, along with oral glucose-lowering medications. In individuals with impaired insulin secretion, often due to β-cell destruction, administration of exogenous insulin may also be necessary for treatment (Basevi, 2011).
Maintaining optimal blood glucose levels is of paramount importance in the treatment of metabolic diseases such as diabetes. Research suggests that eating foods with low sugar levels that are rich in fibre is associated with a lower risk of developing type 2 diabetes. Carbohydrates with a sweet flavour but a minimal impact on blood sugar levels may be healthier options compared to simple sugars such as sucrose. Despite their sweetness, simple sugars are notorious for causing rapid and substantial spikes in blood sugar levels after meals (Mäki‐Arvela et al., 2011).
The food industry is greatly interested in innovative functional ingredients that can reduce the glycaemic effect of sugary foods while retaining their sweet taste and other important properties such as texture. There is currently considerable interest in L-arabinose, a pentose derived from plant materials through enzymatic hydrolysis, such as hemicellulose from sugar beets. Sugar beets yield large amounts of polysaccharide, including arabinan, a hemicellulose consisting of L-arabinose groups linked via (1.3)-α- or (1,2)-α-linkages or both. When sugar beet pulp is treated with aqueous lime, arabinan can be hydrolysed to produce L-arabinose (Mäki‐Arvela et al., 2011). It was found that glucose and serum insulin spikes were both significantly reduced when consuming a sucrose based meal containing 4% arabinose (Inoue et al., 2000). The same also occurs when co-ingesting L-arabinose and glucose, reducing both peak plasma glucose concentration as well as post-prandial concentrations (Pasmans et al., 2022).
L-arabinose is known to prevent the breakdown of sucrose in the brush border of the small intestine by uncompetitive inhibition of the enzyme sucrase. This enzyme is responsible for breaking down sucrose to glucose and fructose which are more easily absorbed by the body. L-arabinose accomplishes this through strongly binding to intestinal surcrase-sucrose complex to form a triple enzyme-substrate-inhibitor complex with low sucrase activity (Seri et al., 1996). As the binding of L-arabinose is so strong, it is able to remain bound for several hours. Therefore one benefit of consuming L-arabinose together with sucrose could be that the digestion of sucrose is delayed resulting in a slower absorption of glucose. Consequently, this delay may lead to a lower glucose and insulin response (Pol et al., 2022).
Figure 3. Diagram of sucrase mode of action. The enzyme binds to sucrose at the active site to form an enzyme-substrate complex. This induces hydrolysis of the carbohydrate into glucose and fructose. If L-arabinose binds to the enzyme-substrate complex to form an enzyme-substrate-inhibitor complex, sucrase’s activity will be inhibited and sucrose will not broken down into glucose and fructose.
Interestingly, when testing the metabolism of L-arabinose no study so far has indicated that any participants experienced gastrointestinal stress or other side effects in humans. This promising finding may lead to the adoption of L-arabinose as a treatment option for type 2 diabetes and related disorders. Currently, a drug named acarbose is used to treat the disease. The antidiabetic drug acts as a starch inhibitor and prevents alpha glucosidases from functioning (Lobato et al., 2024). These enzymes are normally essential for the digestion of various complex carbohydrates however the drug currently induces gastrointestinal stress, flatulence, diarrhoea and more. All of which may be alleviated if L-arabinose was a viable treatment option. The most likely reason as to why L-arabinose does not have as many adverse effects is due to its specificity to sucrase. In contrast acarbose has many non-specific effects. Although promising more research is required before L-arabinose is a viable option for patients (Rosak & Mertes, 2012; Lobato et al., 2024).
In another study conducted by Krog-Mikkelsen et al. (2011) using Caco-2 human cell line, which closely replicates the intestinal mucosa, it was observed that the consumption of L-arabinose led to elevated levels of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1). These hormones are associated with causing increased insulin secretion.
As of 2021, only a limited number of human studies have been conducted investigating L-arabinose and its effects on glucose regulation. These studies primarily found that supplementing sucrose with L-arabinose in water tended to attenuate the post-prandial blood glucose and insulin response. The doses in these studies, which were conducted with beverages and gels, varied from 0.9 g L-arabinose per 30 g sucrose to 15 g L-arabinose per 35 g sucrose. The influence of L-arabinose intake in realistic food containing typical amounts of sucrose has not been extensively studied. This led Pol and Mars (2021) to investigate the glycaemic and insulinemic responses in human subjects to L-arabinose added to realistic liquid and solid sucrose-containing foods, specifically fruit-based drinks and muffins.
Pol and Mars (2021) conducted a double-blind, randomised, cross-over study with five treatments, categorising subjects into two groups: drinks and food. Participants were initially randomised to receive three drinks (Experiment A), including a control, L-arabinose and another drink (D-xylose), followed by two muffins (Experiment B), namely the control muffin and the L-arabinose muffin. Each test food contained approximately 10 g of L-arabinose per 100 g sucrose. The study involved 15 healthy male volunteers recruited from Wageningen (The Netherlands) and surrounding areas, meeting specific eligibility criteria, including being male, healthy, aged between 18 and 35 years, having normal body weight (body mass index (BMI) 18.5 to 25 kg/m2), stable body weight (no weight loss or gain of >5 kg in the 2 months prior to screening), normal fasting glucose ( <6.1 mmol/L) and normal haemoglobin concentration (fasting Hb >8.5 mmol/L).
Participants, after fasting overnight, provided a baseline venous blood sample before consuming either drinks or muffins over a specified period. Blood and breath samples, along with appetite questionnaires were collected at specific intervals following consumption. Glycaemic and insulinemic responses were evaluated, considering maximum concentration (Cmax), time to peak, and incremental area under the curve (iAUC) of plasma glucose and insulin.
The addition of L-arabinose to drinks led to a reduction in both glycaemic and insulin peaks compared to control drinks. Unlike the control, where plasma glucose spiked quickly and declines rapidly, L-arabinose induced more gradual rises and declines. At individual time points, lower glucose concentrations were observed after consuming L-arabinose compared to the control. Similarly, the insulin response with L-arabinose was less abrupt, showing lower concentrations, along with a significantly reduced peak insulin concentration. These findings align with previous studies, such as Inoue et al. (2000) and Krog-Mikkelsen et al. (2011), which demonstrated a decrease in glucose and insulin levels with the addition of L-arabinose to sugar-containing beverages.
Furthermore, Pol and Mars (2021) explored the impact of L-arabinose on solid food, specifically muffins, finding somewhat inconclusive results. While L-arabinose did not notably reduce the glycaemic peak or response in the muffins, there was a tendency for a lower insulin response 30 minutes post-consumption compared to the control muffin, indicating a somewhat diminished overall insulin response. However, no other discernible differences were observed in glycaemic and insulin responses between the L-arabinose and control muffins. The authors speculated that the moderate effect observed could be due to the solid food form and its complex nutrient composition, which include fat, starch, and protein, potentially influencing gastric emptying rate and glycaemic load. Additionally, the inhibitory effect of L-arabinose on maltase activity, proposed by previous studies, might have been less potent in this context (Halschou-Jensen et al., 2014 : Jurgoński et al., 2015).
The potential role of L-arabinose dietary supplementation in the maintenance of glucose and insulin homeostasis demonstrated by Pol and Mars (2021) is supported by Shen et al. (2021)’s work, which involved the investigation of L-arabinose’s effects on high-fat diet/streptozotocin-induced T2DM mice. These effects were found to be antidiabetic, and included: a reduction in blood glucose levels, the regulation of glucose tolerance and dyslipidaemia, an improvement in indicators of steatosis, reduced oxidative damage, and an increase in the anti-inflammatory IL-10 cytokine accompanied by a decrease in the pro-inflammatory IL-6 and IL-1β cytokines. Furthermore, a decrease in the Firmicutes/Bacteroidetes ratio was observed in the faeces of L-arabinose-treated mice, indicating that L-arabinose may exert therapeutic effects in T2DM by modulating the composition of the gut microbiome (Shen et al., 2021).
L-Arabinose and the Gut Microbiome
L-Arabinose’s Effect on the Gut Microbiome and Its Implications for Human Health
In addition to its direct effects on human physiology and metabolism, L-arabinose has also been found to influence the health of mammalian hosts by altering the composition and metabolic activity of the gut microbiome. In the following sections, the relationship between the gut microbiota and human hosts in health and disease, the manipulation of gut microbiome composition by means of probiotics and prebiotics, L-arabinose as a prebiotic and the effect of short chain fatty acids (SCFAs) on host physiology and metabolism will be discussed.
Human Gut Microbiome
The human gut microbiome refers to the large microbial community, with a population of up to 100 trillion microbes, that resides within the adult human intestine, outnumbering human somatic and germ cells by approximately 10 times. The relationship between the human host and the gut microbiota can be considered to be mutualistic, with both parties acquiring increased fitness (Bäckhed et al., 2005). The analysis of 16S rDNA sequences from mucosal tissue and faecal samples obtained from three healthy human adults was carried out to characterise the microflora of the human intestine. Most of the bacterial organisms were found to belong to either the Firmicutes or Bacteroidetes phyla whereas the single archaeal phylotype was found to be the methanogenic Methanobervibacter smithii (M. smithii) (Eckburg et al., 2005).
The gut microbiome was found to possess an abundance of genes involved in the metabolism of starch, sucrose, glucose, fructose, galactose, xylose, mannose, and arabinose, with a minimum of 81 glycoside hydrolase families being represented. A substantial number of these glycoside hydrolases are absent within the human glycobiome. Furthermore, an enrichment of genes involved in the production of short chain fatty acids (SCFAs), which are products of the fermentation of microbiota-accessible carbohydrates (MACs), and in the methanogenic pathway responsible for the removal of the H2 fermentation product, was observed. (Gill et al., 2006). Therefore, one of the ways in which the human host benefits from the presence of the gut microbiota is the microbes’ ability to degrade polysaccharides that are indigestible to the host, making the energy stored within such dietary foods available for use (Bäckhed et al., 2005).
Selectivity in the Gut Microflora’s Utilisation of Polysaccharides
In 1977, Salyers et al. systematically investigated the ability of Bacteroides species found in the human distal gut to utilise dietary plant and host-derived polysaccharides of differing linkages and components. Polysaccharide utilisation differed between Bacteroides groups of different DNA homology, with Bacteroides thetaiotaomicron (B. thetaiotaomicron) and Bacteroides ovatus (B. ovatus) having the widest variety of fermentable substrates (Salyers et al., 1977). This is indicative of microbiome constituents preferably fermenting certain polysaccharides over others, a notion that has been supported in subsequent studies (Martens et al., 2011; Sonnenburg et al., 2010). This selective use of dietary polysaccharides by gut microbiota suggests the possibility of modulating populations of microbiome constituents by means of dietary supplements that encourage the growth of particular microbes over others. This forms the basis for the concept of “prebiotics”, which will be discussed in a following section.
Figure 4. Schematic illustration of carbohydrate fermentation by intestinal microbes (Macfarlane & Macfarlane, 2003).
Disease-Associated Alterations in Gut Microbiome Composition
The gut microbiome constituents of healthy individuals have been found to differ from those of individuals with metabolic diseases, such as obesity, or inflammatory bowel disease (IBD) (Ley et al., 2005; Frank et al., 2007). For example, the obesity-associated alterations in gut microbiome composition were investigated using DNA from the caecum of obese mice and their lean siblings. The obesity phenotype was found to be associated with a shift in community structure at the division level, with obese mice exhibiting a substantially larger proportion of Firmicutes and a 50% reduction in Bacteroidetes in comparison to lean mice (Ley et al., 2005). The increased Firmicutes/Bacteroidetes ratio in obese mice was also observed in Turnbaugh et al. (2006)’s study. These differences in gut microbiota proportions in individuals in a diseased state, in comparison to individuals in a healthy state, indicate that the modulation of such proportions to more closely resemble the healthy microbial community may be an effective therapeutic strategy.
Manipulation of Gut Microbiome Composition via Pro- and Prebiotics
Probiotics and prebiotics are two kinds of supplements that can be used to restore balance to the intestinal microflora in order to improve the health of the host animal (Gibson & Roberfroid, 1995). In the case of the former, the supplement would consist of either a single or multiple live strains of bacteria whose introduction into the host would have beneficial effects on the gut microbiome and consequently on the host (Fuller, 1989). On the other hand, prebiotics are nondigestible dietary elements that improve the balance of intestinal microflora by selectively promoting the growth and metabolic activity of specific microbial members of the gut microbiome, with an overall beneficial effect on the host (Gibson & Roberfroid, 1995). Cani et al. (2007b)’s study is a good example of the use of prebiotics. Bifidobacteria species were found to be depleted in the intestinal microbiota of mice that were fed a high-fat diet and this decrease in Bifidobacteria, as well as other bacteria, was accompanied by higher endotoxemia (Cani et al., 2007a). Based on these findings, the authors sought to specifically increase the population of Bifidobacteria in the intestine of mice fed a high-fat diet by supplementing the diet with a prebiotic, oligofructose. This resulted in a restoration of the Bifidobacterial population and in an associated normalisation of endotoxemia to control levels (Cani et al., 2007b). Similarly, L-Arabinose has been found to beneficially alter the composition of the gut microbiome in several mouse studies, some of which will be discussed in the following section.
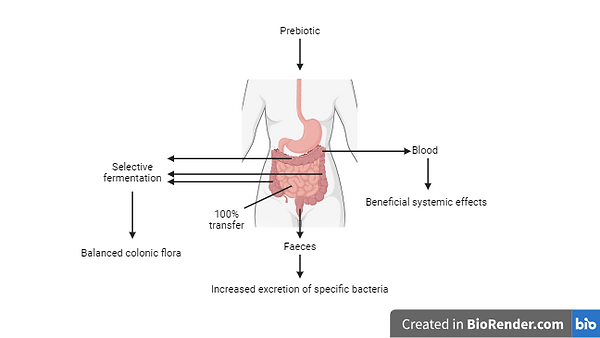
Figure 5. The selective fermentation of prebiotics by gut microbes improves the balance of the gut microbiota, resulting in health benefits for the host organism. Furthermore, the absorption of fermentation products into the host organism’s bloodstream may have further beneficial systemic effects (from Gibson & Roberfroid, 1995).
Arabinose as a Prebiotic
Isoflavone Metabolism
In a study by Tamura et al. (2012), the effect of L-arabinose supplementation on mouse intestinal microbiota and their ability to metabolise the isoflavone daidzein into equol, which has been found to be linked to a decreased prostate cancer incidence, was investigated (Akaza et al., 2004; Tamura et al., 2012). Two groups of seven male mice each were utilized in the experiment, with one group being fed a control diet with 0.05% daidzein, whereas the other was fed the test diet containing 0.05% daidzein and 2.5% L-arabinose for 28 days. The equol/daidzein ratio was found to be significantly higher within the arabinose test group in comparison to the control group. Moreover, differences in the two groups’ gut microbiota were also noted, with the arabinose group having higher occupation ratios of SCFA-producing Bacteroides and Bifidobacteria than the control group. It was suggested that this L-arabinose-induced change in intestinal microbiota composition increased daidzein metabolism into equol in the arabinose test group (Tamura et al., 2012).
Metabolic Syndrome
L-arabinose was also found to ameliorate various aspects of metabolic syndrome in rats by causing: a decrease in serum triglycerides, total cholesterol, low-density lipoprotein cholesterol (LDL-C), free fatty acids and an increase in high-density lipoprotein cholesterol (HDL-C); the normalization of GLUT4 levels and the resultant reduction in insulin resistance; anti-inflammatory changes in cytokine expression; and an improvement in visceral organ function (Hao et al., 2015). Similar findings were obtained by Zhao et al. (2019), who also investigated the role of L-arabinose in altering gut microbiota proportions and activity, particularly concerning the production of intestinal hydrogen gas production. L-arabinose gavage into mice was found to induce the gut microbiota-mediated production of hydrogen gas in a dose- and time-dependent manner, as this effect was lost upon treatment with antibiotics. The analysis of faecal 16S rRNA gene content by q-PCR revealed an alteration in the balance of intestinal hydrogen gas-producing and -consuming microbiota in response to L-arabinose dietary supplementation. In fact, L-arabinose was found to decrease the abundance of the hydrogen-consuming M. smithii and Desulfovibrios. Furthermore, L-arabinose treatment restored the disturbed Firmicutes/Bacteroidetes ratio in high fat diet mouse models to normal levels observed in the control group (Zhao et al., 2019).
Probiotic Effects of L-Arabinose
The beneficial effects of L-arabinose on host physiology that were observed in the above studies were supported by Xiang et al. (2024) in their study, as the introduction of L-arabinose into a variety of diet types was associated with reduced liver weight, and reduced diet-induced inflammation. The effect of L-arabinose on the colonic microbiota was also investigated, and its main probiotic effect was determined to be the elevated abundance of Bifidobacteria from the Actinobacteria phylum, and the increase in intestinal SCFA production.
SCFA’s Effects on Host Physiology and Metabolism via GPR41 and GPR43
The L-arabinose-induced increase in intestinal SCFA production by the gut microbiota may have systemic effects on host physiology metabolism, as in 2003, Brown et al. identified the cognate ligands of two G protein-coupled receptors, GPR41 and GPR43, as being SCFAs, with the two receptors having different specificities for the SCFA’s carbon chain length (Figure 6). Both GPR41 and GPR43 were found to activate Gi/o family proteins, however only GPR43 generated an intracellular calcium response to acetate that was resistant to pertussis toxin, indicating its coupling to Gq (Brown et al., 2003). These receptors have been found to be expressed on adipocytes, polymorphonuclear cells, as well as enteroendocrine cells, with their SCFA-mediated activation having a variety of effects, some of which have been summarised in Table 1 and 2 (Hong et al., 2005, Le Poul et al., 2003; Tolhurst et al., 2012).
Figure 6. Microbiota-accessible carbohydrates from plant-based foods are digested by gut microbes, releasing L-arabinose, which is then fermented by bacteria to yield SCFA products. These SCFAs can then enter the host’s circulation and can influence the host’s metabolism and physiology by binding to and activating SCFA-activated GPCRs, GPR41 and GPR43 (adapted from Sonnenburg & Sonnenburg, 2014).
Table 1. Effects of GPR43 activation via SCFA
Table 2. Effects of GPR41 activation via SCFA
Conclusion
In conclusion, L-arabinose has shown promising potential in relation to human health, particularly in the management of diabetes mellitus, through effects in maintaining blood glucose and insulin homeostasis - a crucial aspect of diabetes management. Furthermore, dietary supplementation with L-arabinose has also been associated with improvements in metabolic syndrome parameters within rats, such as decreased serum triglycerides and total cholesterol, as well as reductions in insulin resistance and diet-induced inflammation. Its probiotic effects, including the promotion of beneficial bacteria, and the production of short-chain fatty acids, further highlight its therapeutic potential. Despite such findings, the mechanisms of L-arabinose metabolism in humans still remains poorly understood, with limited human studies conducted thus far.
Overall, existing evidence and research suggests that L-arabinose holds promise within the pharmaceutical and biotechnological industries. Further exploration of such potential within human health is warranted, particularly in elucidating the underlying metabolic mechanisms.




Overall Effect | Mechanism or Evidence | Reference |
|---|---|---|
Inflammation | Acetate fed to dextran sulphate sodium (DSS)-induced colitis mice models reduced inflammation in such mice in a GPR43-dependent manner, as GPR43 knockout mice experienced none of the beneficial effects exhibited in wild-type mice.
Inflammation was also observed to be worse in GPR43 knockout inflammatory arthritis and allergic airway inflammation model mice, in comparison to wild-type mice. | Maslowski et al., 2009 |
Stimulation of glucagon-like peptide-1 (GLP-1) secretion | The activation of GPR43 by SCFAs resulted in elevated intracellular Ca2+ levels within enteroendocrine L cells, most likely via coupling to Gq signalling pathways, to stimulate GLP-1 secretion.
| Tolhurst et al., 2012 |
Suppression of fat accumulation | In adipocytes, SCFA-induced GPR43 activation results in the suppression of insulin signalling-mediated fat accumulation via the Gi/oβγ -Phospholipase C - protein kinase C - phosphatase and tensin homolog (PTEN) pathway, which results in decreased Akt phosphorylation.
GPR43 was also found to increase insulin sensitivity and energy expenditure in liver and muscle tissue.
| Kimura et al., 2013
|
Stimulation of adipogenesis and inhibition of lipolysis | GPR43 was found to be upregulated in adipose tissues of mice fed a high-fat diet.
Acetate and propionate activate GPR43 to stimulate adipocyte differentiation, fat accumulation and the inhibition of lipolysis.
Activation of GPR43 by acetate and propionate in adipocytes activates the coupled Gi pathway and results in the inhibition of lipolysis and reduction of plasma free fatty acid levels. | Hong et al., 2005;
Ge et al., 2008 |
Overall Effect | Mechanism or Evidence | Reference |
|---|---|---|
Sympathetic Nervous System | The propionate-mediated activation of GPR41 expressed on sympathetic ganglia resulted in the activation of sympathetic neurons by means of Gβγ – Phospholipase C-β (PLC-β) - mitogen-activated protein kinase (MAPK) signalling.
| Kimura et al., 2011 |
Host adiposity | Activation of GPR41 expressed in enteroendocrine cells by microbiota-produced SCFAs resulted in the increase of circulating peptide YY levels in B. thetaiotaomicron and M. smithii-colonised GPR41 wild-type mice. This was accompanied by a decreased intestinal transit rate, and an increased absorption of SCFAs for use as lipogenesis substrates in the liver.
GPR41 knockout male mice were found to develop an increased mass of body fat when given a low- or high-fat diet, in comparison to wild-type male and female mice. The suspected cause was determined to be reduced energy expenditure. | Samuel et al., 2008;
Bellahcene et al., 2013
|
References
Adamczyk, P. A., Coradetti, S. T., & Gladden, J. M. (2023). Non-canonical D-xylose and L-arabinose metabolism via D-arabitol in the oleaginous yeast Rhodosporidium toruloides. Microbial Cell Factories, 22(1), 145. 10.1186/s12934-023-02126-x
Akaza, H., Miyanaga, N., Takashima, N., Naito, S., Hirao, Y., Tsukamoto, T., Fujioka, T., Mori, M., Kim, W. J., Song, J. M., & Pantuck, A. J. (2004). Comparisons of percent equol producers between prostate cancer patients and controls: case-controlled studies of isoflavones in Japanese, Korean and American residents. Japanese journal of clinical oncology, 34(2), 86–89. https://doi.org/10.1093/jjco/hyh015
Asatkar, A. K., & Basak, R. K. (2023). Chapter 2 - Carbohydrate: Introduction and fundamentals. Handbook of Biomolecules, 25-55. https://doi.org/10.1016/B978-0-323-91684-4.00020-7
Bäckhed, F., Ley, R. E., Sonnenburg, J. L., Peterson, D. A., & Gordon, J. I. (2005). Host-bacterial mutualism in the human intestine. Science (New York, N.Y.), 307(5717), 1915–1920. https://doi.org/10.1126/science.1104816
Bailes, B. K. (2002). Diabetes Mellitus and its Chronic Complications. AORN Journal, 76(2), 265–282. https://doi.org/10.1016/s0001-2092(06)61065-x
Basevi, V. (2011). Diagnosis and classification of diabetes mellitus. Diabetes Care, 34(Supplement_1), S62–S69. https://doi.org/10.2337/dc11-s062
Bellahcene, M., O'Dowd, J. F., Wargent, E. T., Zaibi, M. S., Hislop, D. C., Ngala, R. A., Smith, D. M., Cawthorne, M. A., Stocker, C. J., & Arch, J. R. S. (2013). Male mice that lack the G-protein-coupled receptor GPR41 have low energy expenditure and increased body fat content. British Journal of Nutrition, 109(10), 1755-1764. https://doi.org/10.1017/S0007114512003923
Brown, A. J., Goldsworthy, S. M., Barnes, A. A., Eilert, M. M., Tcheang, L., Daniels, D., Muir, A. I., Wigglesworth, M. J., Kinghorn, I., Fraser, N. J., Pike, N. B., Strum, J. C., Steplewski, K. M., Murdock, P. R., Holder, J. C., Marshall, F. H., Szekeres, P. G., Wilson, S., Ignar, D. M., Foord, S. M., … Dowell, S. J. (2003). The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. The Journal of biological chemistry, 278(13), 11312–11319. https://doi.org/10.1074/jbc.M211609200
Cani, P. D., Amar, J., Iglesias, M. A., Poggi, M., Knauf, C., Bastelica, D., Neyrinck, A. M., Fava, F., Tuohy, K. M., Chabo, C., Waget, A., Delmée, E., Cousin, B., Sulpice, T., Chamontin, B., Ferrières, J., Tanti, J., Gibson, G. R., Casteilla, L., . . . Burcelin, R. (2007a). Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes, 56(7), 1761-1772. https://doi.org/10.2337/db06-1491
Cani, P. D., Neyrinck, A. M., Fava, F., Knauf, C., Burcelin, R. G., Tuohy, K. M., Gibson, G. R., & Delzenne, N. M. (2007b). Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia, 50(11), 2374–2383. https://doi.org/10.1007/s00125-007-0791-0
Crozier, L., Marshall, J., Holmes, A., Wright, K. M., Rossez, Y., Merget, B., Humphris, S., Toth, I., Jackson, R. W., & Holden, N. J. (2021). The role of l-arabinose metabolism for Escherichia coli O157:H7 in edible plants. Microbiology (Society for General Microbiology), 167(7)https://doi.org/10.1099/mic.0.001070
Eckburg, P. B., Bik, E. M., Bernstein, C. N., Purdom, E., Dethlefsen, L., Sargent, M., Gill, S. R., Nelson, K. E., & Relman, D. A. (2005). Diversity of the human intestinal microbial flora. Science (New York, N.Y.), 308(5728), 1635–1638. https://doi.org/10.1126/science.1110591
Fehér, C. (2018). Novel approaches for biotechnological production and application of L-arabinose. Journal of Carbohydrate Chemistry, 37(5), 251-284. https://doi.org/10.1080/07328303.2018.1491049
Frank, D. N., St Amand, A. L., Feldman, R. A., Boedeker, E. C., Harpaz, N., & Pace, N. R. (2007). Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proceedings of the National Academy of Sciences of the United States of America, 104(34), 13780–13785. https://doi.org/10.1073/pnas.0706625104
Fujii, M., Hatozoe, M., Hou, D., Sanada, H., Osaki, S., & Hizukuri, S. (2000). Effects of L-Arabinose on Serum Neutral Lipid, Weights of Fat Pads and Cecum, and on Organic Acids in Cecum in Rats. Journal of Applied Glycoscience, 47(3-4), 355-361. 10.5458/jag.47.355
Ge, H., Li, X., Weiszmann, J., Wang, P., Baribault, H., Chen, J., Tian, H., & Li, Y. (2008). Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology, 149(9), 4519-4526. https://doi.org/10.1210/en.2008-0059
Gibson, G. R., & Roberfroid, M. B. (1995). Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. The Journal of Nutrition, 125(6), 1401-1412. https://doi.org/10.1093/jn/125.6.1401
Gill, S. R., Pop, M., Deboy, R. T., Eckburg, P. B., Turnbaugh, P. J., Samuel, B. S., Gordon, J. I., Relman, D. A., Fraser-Liggett, C. M., & Nelson, K. E. (2006). Metagenomic analysis of the human distal gut microbiome. Science (New York, N.Y.), 312(5778), 1355–1359. https://doi.org/10.1126/science.1124234
Halschou-Jensen, K., Knudsen, K. E. B., Nielsén, S., Bukhave, K., & Andersen, J. R. (2014). A mixed diet supplemented with l-arabinose does not alter glycaemic or insulinaemic responses in healthy human subjects. British Journal of Nutrition, 113(1), 82–88. https://doi.org/10.1017/s0007114514003407
Hao, L., Lu, X., Sun, M., Li, K., Shen, L., & Wu, T. (2015). Protective effects of L-arabinose in high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. Food & nutrition research, 59, 28886. https://doi.org/10.3402/fnr.v59.28886
Hong, Y., Nishimura, Y., Hishikawa, D., Tsuzuki, H., Miyahara, H., Gotoh, C., Choi, K., Feng, D. D., Chen, C., Lee, H., Katoh, K., Roh, S., & Sasaki, S. (2005). Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology, 146(12), 5092-5099. https://doi.org/10.1210/en.2005-0545
Inoue, S., Sanai, K., & Seri, K. (2000). Effect of L-arabinose on blood glucose level after ingestion of sucrose-containing food in human. Nihon Eiyō, Shokuryō Gakkai Shi, 53(6), 243-247. 10.4327/jsnfs.53.243
Jurgoński, A., Krotkiewski, M., Juśkiewicz, J., & Billing-Marczak, K. (2015). Suppression of Postprandial Glycaemia by L-Arabinose in Rats is More Associated with Starch Than Sucrose Ingestion - a Short Report. Polish Journal of Food and Nutrition Sciences, 65(1), 57–60. https://doi.org/10.1515/pjfns-2015-0001
Kaul, K., Tarr, J. M., Ahmad, S., Kohner, E. M., & Chibber, R. (2012). Introduction to diabetes mellitus. In Advances in Experimental Medicine and Biology (pp. 1–11). https://doi.org/10.1007/978-1-4614-5441-0_1
Kharroubi, A., & Darwish, H. M. (2015). Diabetes mellitus: The epidemic of the century. World Journal of Diabetes, 6(6), 850. https://doi.org/10.4239/wjd.v6.i6.850
Kimura, I., Inoue, D., Maeda, T., Hara, T., Ichimura, A., Miyauchi, S., Kobayashi, M., Hirasawa, A., & Tsujimoto, G. (2011). Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proceedings of the National Academy of Sciences of the United States of America, 108(19), 8030–8035. https://doi.org/10.1073/pnas.1016088108
Kimura, I., Ozawa, K., Inoue, D., Imamura, T., Kimura, K., Maeda, T., Terasawa, K., Kashihara, D., Hirano, K., Tani, T., Takahashi, T., Miyauchi, S., Shioi, G., Inoue, H., & Tsujimoto, G. (2013). The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nature Communications, 4(1), 1829. https://doi.org/10.1038/ncomms2852
Krog-Mikkelsen, I., Hels, O., Tetens, I., Juul Holst, J., Rikardt Andersen, J., & Bukhave, K. (2011). The effects of l-arabinose on intestinal sucrase activity: dose-response studies in vitro and in humans. The American Journal of Clinical Nutrition, 94(2), 472-478. 10.3945/ajcn.111.014225
Le Poul, E., Loison, C., Struyf, S., Springael, J., Lannoy, V., Decobecq, M., Brezillon, S., Dupriez, V., Vassart, G., Van Damme, J., Parmentier, M., & Detheux, M. (2003). Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation*. Journal of Biological Chemistry, 278(28), 25481-25489. https://doi.org/10.1074/jbc.M301403200
Ley, R. E., Bäckhed, F., Turnbaugh, P., Lozupone, C. A., Knight, R. D., & Gordon, J. I. (2005). Obesity alters gut microbial ecology. Proceedings of the National Academy of Sciences of the United States of America, 102(31), 11070–11075. https://doi.org/10.1073/pnas.0504978102
Lobato, C. B., Winding, C. T., Bojsen-Møller, K. N., Martinussen, C., Veedfald, S., Holst, J. J., Madsbad, S., Jørgensen, N. B., & Dirksen, C. (2024). Canagliflozin or acarbose versus placebo to ameliorate post-bariatric hypoglycaemia - The HypoBar I randomized clinical trial protocol. Diabetic Medicine, e15320. 10.1111/dme.15320
Macfarlane, S., & Macfarlane, G. T. (2003). Regulation of short-chain fatty acid production. The Proceedings of the Nutrition Society, 62(1), 67–72. https://doi.org/10.1079/PNS2002207
Mäki‐Arvela, P., Salmi, T., Holmbom, B., Willför, S., & Murzin, D. Y. (2011). Synthesis of sugars by hydrolysis of hemicelluloses- a review. Chemical Reviews, 111(9), 5638–5666. https://doi.org/10.1021/cr2000042
Martens, E. C., Lowe, E. C., Chiang, H., Pudlo, N. A., Wu, M., McNulty, N. P., Abbott, D. W., Henrissat, B., Gilbert, H. J., Bolam, D. N., & Gordon, J. I. (2011). Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLOS Biology, 9(12), e1001221. https://doi.org/10.1371/journal.pbio.1001221
Maslowski, K. M., Vieira, A. T., Ng, A., Kranich, J., Sierro, F., Yu, D., Schilter, H. C., Rolph, M. S., Mackay, F., Artis, D., Xavier, R. J., Teixeira, M. M., & Mackay, C. R. (2009). Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature, 461(7268), 1282-1286. https://doi.org/10.1038/nature08530
Mojzita, D., Penttilä, M., & Richard, P. (2010). Identification of an L-arabinose reductase gene in Aspergillus niger and its role in L-arabinose catabolism. The Journal of Biological Chemistry, 285(31), 23622-23628. 10.1074/jbc.M110.113399
Ogurtsova, K., Fernandes, J., Huang, Y., Linnenkamp, U., Guariguata, L., Cho, N. H., Cavan, D., Shaw, J. E., & Makaroff, L. (2017). IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Research and Clinical Practice, 128, 40–50. https://doi.org/10.1016/j.diabres.2017.03.024
Onkenhout, W., Groener, J. E. M., Verhoeven, N. M., Yin, C., & Laan, L. A. E. M. (2002). l-Arabinosuria: a new defect in human pentose metabolism. Molecular Genetics and Metabolism, 77(1), 80-85. 10.1016/S1096-7192(02)00125-7
Pasmans, K., Meex, R. C. R., Trommelen, J., Senden, J. M. G., Vaughan, E. E., van Loon, L. J. C., & Blaak, E. E. (2022). L-arabinose co-ingestion delays glucose absorption derived from sucrose in healthy men and women: a double-blind, randomised crossover trial. British Journal of Nutrition, 128(6), 1072-1081. 10.1017/S0007114521004153
Petersmann, A., Müller‐Wieland, D., Müller, U., Landgraf, R., Nauck, M., Freckmann, G., Heinemann, L., & Schleicher, E. (2014). Definition, Classification and diagnosis of diabetes mellitus. Experimental and Clinical Endocrinology & Diabetes, 122(07), 384–386. https://doi.org/10.1055/s-0034-1366278
Pol, K., De Graaf, K., Bruin, M. D., Balvers, M. G., & Mars, M. (2020). The effect of replacing sucrose with L-arabinose in drinks and cereal foods on blood glucose and plasma insulin responses in healthy adults. Journal of Functional Foods, 73, 104114. https://doi.org/10.1016/j.jff.2020.104114
Pol, K., Puhlmann, M., & Mars, M. (2022). Efficacy of L-Arabinose in lowering glycemic and insulinemic responses: the modifying effect of starch and fat. Foods, 11(2), 157. https://doi.org/10.3390/foods11020157
Puchulu, F. (2017). Definition, Diagnosis and classification of diabetes mellitus. In Springer eBooks (pp. 7–18). https://doi.org/10.1007/978-3-319-72475-1_2
Salyers, A. A., Vercellotti, J. R., West, S. E., & Wilkins, T. D. (1977). Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Applied and environmental microbiology, 33(2), 319–322. https://doi.org/10.1128/aem.33.2.319-322.1977
Samuel, B. S., Shaito, A., Motoike, T., Rey, F. E., Backhed, F., Manchester, J. K., Hammer, R. E., Williams, S. C., Crowley, J., Yanagisawa, M., & Gordon, J. I. (2008). Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proceedings of the National Academy of Sciences of the United States of America, 105(43), 16767–16772. https://doi.org/10.1073/pnas.0808567105
Seiboth, B., & Metz, B. (2011). Fungal arabinan and l-arabinose metabolism. Applied Microbiology and Biotechnology, 89(6), 1665-1673. https://doi.org/10.1007/s00253-010-3071-8
Shen, D., Lu, Y., Tian, S., Ma, S., Sun, J., Hu, Q., Pang, X., & Li, X. (2021). Effects of L-arabinose by hypoglycemic and modulating gut microbiome in a high-fat diet- and streptozotocin-induced mouse model of type 2 diabetes mellitus. Journal of food biochemistry, 45(12), e13991. https://doi.org/10.1111/jfbc.13991
Sonnenburg, E. D., & Sonnenburg, J. L. (2014). Starving our microbial self: The deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metabolism, 20(5), 779-786. https://doi.org/10.1016/j.cmet.2014.07.003
Tamura, M., Kurusu, Y., & Hori, S. (2012). Effect of Dietary l-arabinose on the Intestinal Microbiota and Metabolism of Dietary Daidzein in Adult Mice. Bioscience of microbiota, food and health, 31(3), 59–65. https://doi.org/10.12938/bmfh.31.59
Tolhurst, G., Heffron, H., Lam, Y. S., Parker, H. E., Habib, A. M., Diakogiannaki, E., Cameron, J., Grosse, J., Reimann, F., & Gribble, F. M. (2012). Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-Protein–Coupled receptor FFAR2. Diabetes, 61(2), 364-371. https://doi.org/10.2337/db11-1019
Turnbaugh, P. J., Ley, R. E., Mahowald, M. A., Magrini, V., Mardis, E. R., & Gordon, J. I. (2006). An obesity-associated gut microbiome with increased capacity for energy harvest. Nature, 444(7122), 1027–1031. https://doi.org/10.1038/nature05414
Xiang, S., Ge, Y., Zhang, Y., Bao, X., Su, X., Shi, L., Xia, Y., Han, H., Ying, J., Lai, S., Chen, J., & Zhu, X. (2024). L-arabinose exerts probiotic functions by improving gut microbiota and metabolism in vivo and in vitro. Journal of Functional Foods, 113, 106047. https://doi.org/10.1016/j.jff.2024.106047
Zhao, L., Wang, Y., Zhang, G., Zhang, T., Lou, J., & Liu, J. (2019). L-Arabinose Elicits Gut-Derived Hydrogen Production and Ameliorates Metabolic Syndrome in C57BL/6J Mice on High-Fat-Diet. Nutrients, 11(12), 3054. https://doi.org/10.3390/nu11123054



